Written by: <Authors><Author><Id>5429</Id><Name>Lipsa Mohapatra</Name><FriendlyName>lipsa-mohapatra</FriendlyName><AuthorImage>/5429/lipsa.jpg</AuthorImage></Author></Authors>

Written by: <Authors><Author><Id>5429</Id><Name>Lipsa Mohapatra</Name><FriendlyName>lipsa-mohapatra</FriendlyName><AuthorImage>/5429/lipsa.jpg</AuthorImage></Author></Authors>

The main constituent of glass fibre is silica (Silicon dioxide). The raw material for silica is sand or sandstone. Glass is highly amorphous in nature due to heavy networking in its chemical structure.
Major ingredients:
• Lime [Ca(OH)2] – stabilising agent
• Magnesia [MgO] – stabilising agent
• Alumina [Al2O3] – increases the strength and durability of the glass fibre due to more linking capacity
• Soda
• Potash
• Boric oxide – forms the cross linking in the structure which helps to improve strength
Classification of glass fibre
According to the type of composition glass fibre is classified as:
• Glass A (Alkali)
• Glass C (Chemical)
• Glass E (Electrical)
Composition (in per cent)
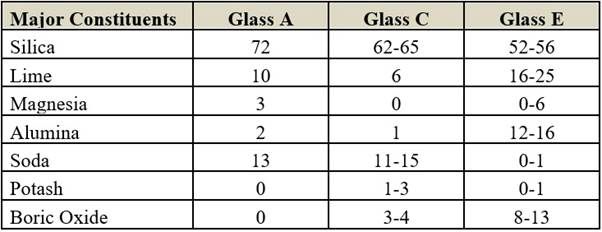
All kinds of glass fibres are highly amorphous in nature. There is no cohesive force between the fibres due to the absence of intermolecular attraction. The more cross-linking capacities of cationic elements like boric oxide and alumina increase the strength of the material.
Glass E is the strongest fibre with high insulation properties. Its moisture regain is only 0.5 per cent. Therefore, there are no polar groups or electrical carriers and so it has high insulating properties. Due to its high strength, it is used for reinforcing purposes. Due to low MR, it has low electrical conductivity.
Production of staple and filament glass fibre
Steps for manufacturing of glass fibre:
• Preparation of glass marbles
• Adding all the ingredients to form a mixture
• Vigorous mixing for homogeneity
• Melting of mixture
• Preparation of marbles of 2 cm diameter
• Melting and extrusion of the filament
• Melting is carried out in an electrical furnace
• High air flow cutting of glass is done during extrusion for creating glass fibres.
• Melting of mixture is done at 8000C.
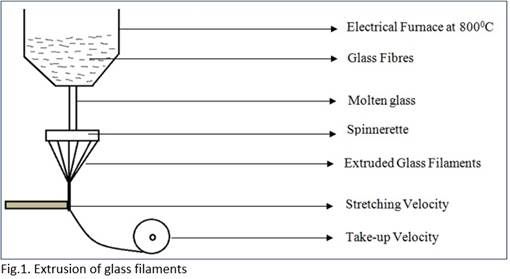
The continuous filament is collected on a take-up roller where, mass flow of extrusion is equal to mass flow of take-up, resulting in conservation of mass. After the filament is drawn, a size is applied depending on the end use. It is then wound on to a bobbin.
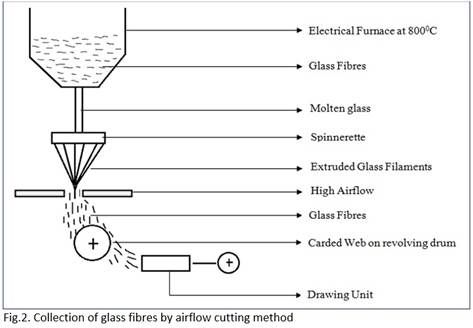
Collection of glass fibres by airflow cutting method:
- It consists of an airflow unit.
- It exerts high airflow on to the material extruded from the Spinnerette.
-This airflow disintegrates the continuity of the filaments.
- The fibres thus formed have varied staple lengths due to airflow cutting.
- The glass fibres are collected as carded web on a revolving drum that directs the web to a drawing unit for sliver formation.

- It has a network of Si-O bonds. Four oxygen atoms surround each silicon atom.
- Irregular structure of the glass fibre
- It has void or interstices, which are filled by different cations like Mg , Al , etc.
- The strength of the glass fibre depends upon
• The % of boric oxide
• The % of alumina
• The ratio of silicon to oxygen bonds (Si:O)
- As valency of Al and B is 3, therefore, they create more cross-linking and hence, impart more strength.
- If Si:O ratio is high, then
• There are more degrees of polymerisation
• Hard glass formation
• Low thermal co-efficient of expansion
- If Si:O ratio is low, then the glass has more softening points.
- Melting of glass helps to detect the last trace of crystallinity present in the glass
- Glass becomes soft when melting of crystalline region starts.
Types of glass products
1. Glass fibres are used for making composites.
2. Glass fibres for electrical insulation and electrical composites (switch board circuits).
Physical and chemical properties of glass fibres
Physical Properties
• Strength depends on Si:O ratio and composition of other cations.
• Density is 2.5-2.6 g/cc
• MP is 750oC
• Highly amorphous
• MR – 0.5 per cent
• Poor abrasion resistance due to its stiff nature
• High Strength
• Dry- 6-10 g/d
• Wet- 5-8 g/d
• Although glass has 0.5 per cent MR capacity, its wet strength is significantly reduced due to the amorphous nature of the fibre.
Chemical Properties
• High resistance to biological agents
• Inert to oxidising agents, heat, and sunlight under normal conditions
• Hydrochloric acid and sulphuric acid can cause hydrolysis and degradation of glass fibre
• Strong alkali or alkali in hot conditions deteriorates or weakens glass fibre
• Due to its hydrophobic nature, it has excellent corrosion resistance
Applications
• Thermal, electrical, and sound insulation
• High strength fabrics
• Heat and corrosion resistant fabrics
• Biomedical application for joint replacement
• Tape (mainly for insulation of motors and cables)
• Braiding (process of preparing long tapes)
• Preparation of composites
• Textured glass yarn (used in making high temperature resistant filters)
• Protective clothing
• Fire retardant clothing
• Industrial fabrics and furnishing fabrics
Reinforcement material for bows and arrows, transparent roof panels, automobile bodies, boat hulls, hockey sticks, surfboards, etc.
References:
1. Hull, D.; Clyne, T. W., eds. (1996), "Fibres and matrices", An Introduction to Composite Materials, Cambridge Solid State Science Series (2 ed.), Cambridge: Cambridge University Press, p. 15, doi:10.1017/cbo9781139170130.004, ISBN 978-1-139-17013-0
2. Loewenstein, K.L. (1973). The Manufacturing Technology of Continuous Glass Fibers. New York: Elsevier Scientific. p. 94. ISBN 978-0-444-41109-9.